Lifileucel
Snippets at a glance
What drug group does it belong to?
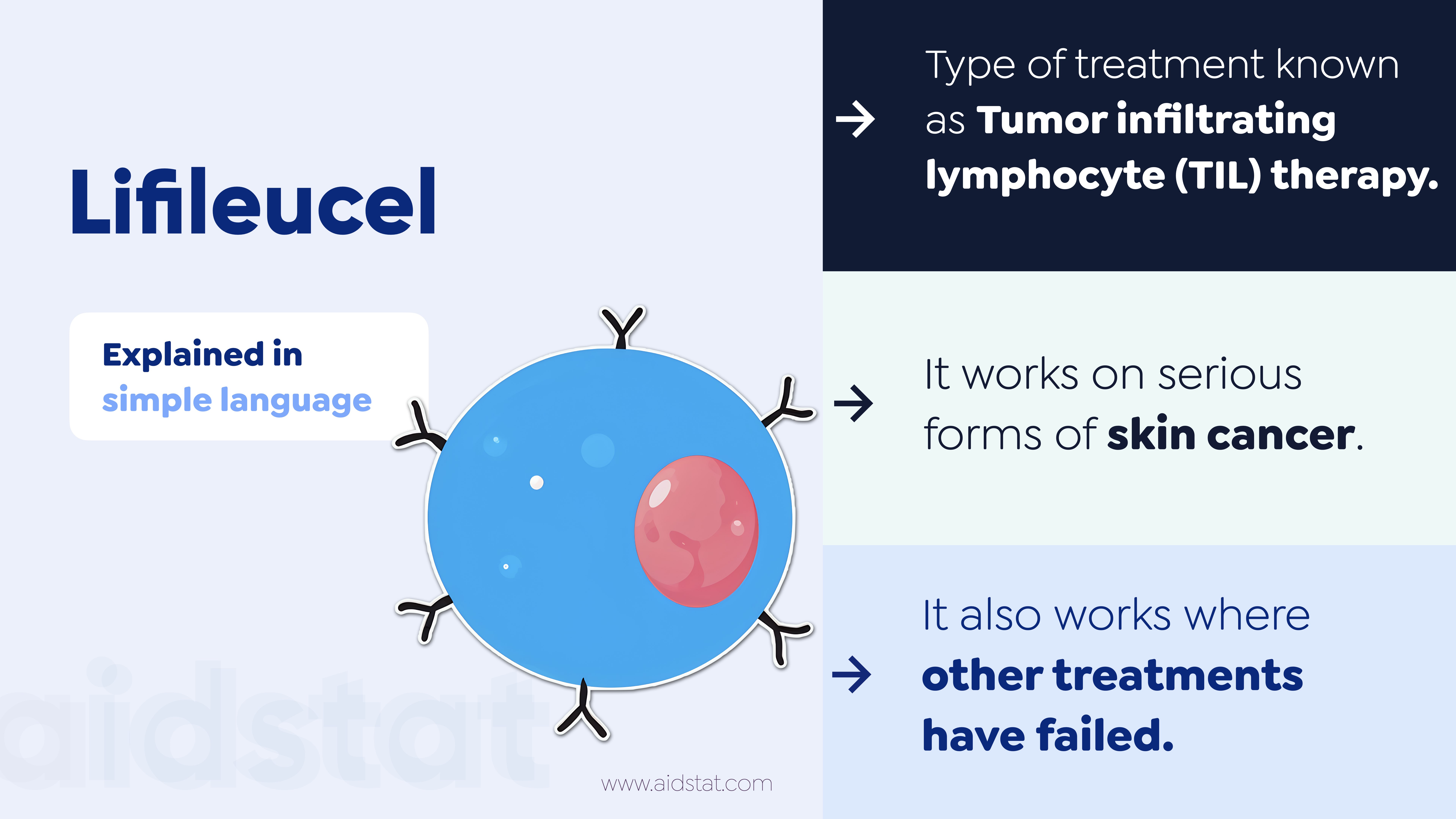
Lifileucel is a "Tumor infiltrating lymphocyte (TIL) therapy."
What does it treat?
It treats adult patients with advanced skin cancer called melanoma.
How does it work?
Patients' own immune cells from the tumor are used. They are taken out, made more effective and put back into the body.

Who shouldn't use it?
Use in pregnancy and active infections is not recommended.
Important prescribing information.
It must only be given in a hospital where intensive care facilities are readily available.
Important patient advice.
Patients should be advised to avoid live vaccines for a few months after.
Table of contents
**Updates**
We updated this drug summary on 7th of June 2024, adding 'Snippets at a glance', removed TLDR section, added simple definitions, and condensed longer paragraphs into summary points to improve readability.

Lifileucel - Tumor infiltrating lymphocyte (TIL) therapy
What is lifileucel used for?
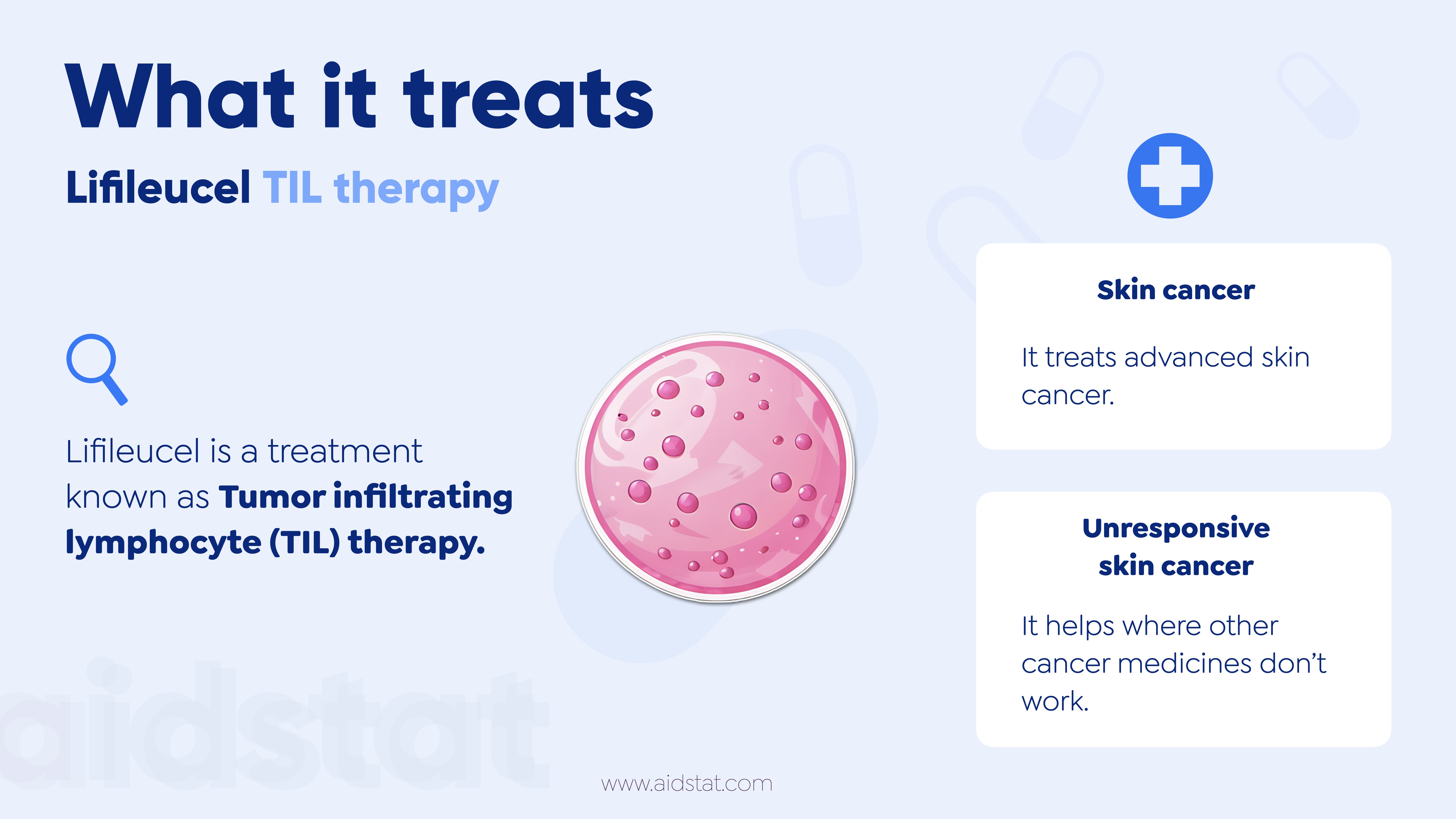
- Lifileucel is a "Tumour infiltrating lymphocyte (TIL)" therapy.
- It uses patients' own immune cells (T cells) from their tumour.
- It treats adult patients with advanced skin cancer called melanoma.
-
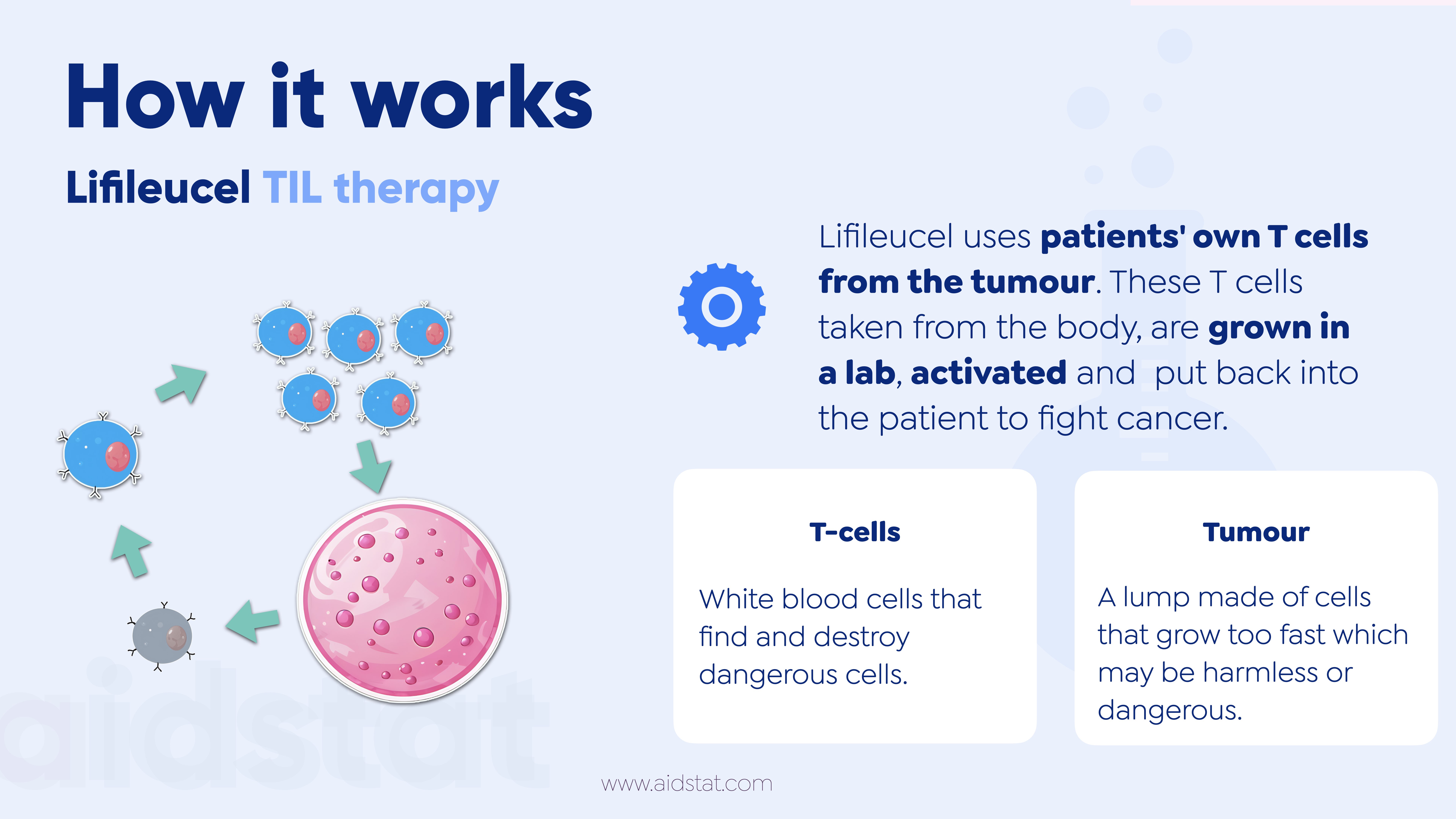
Tumour - A group of cells that grow too fast and form a lump in the body.
-
Lymphocyte - Special blood cells that help our body fight diseases.

Lifileucel - Tumor infiltrating lymphocyte (TIL) therapy
What is the mechanism of action of lifileucel?
- Lifileucel uses patients' own immune cells (T cells) from the tumour.
- These T cells taken from the body, are grown in a lab, activated and put back into the patient to fight cancer.
- Lifileucel is given as a one-time intravenous infusion.
- Patients can start to see effects within 1-2 months after infusion.
-
T cells - White blood cells that find and destroy dangerous cells.
-
Intravenous infusions - Injected directly into veins.

Lifileucel - Tumor infiltrating lymphocyte (TIL) therapy
What are the contraindications of lifileucel?
- How lifileucel affects an unborn baby is not known.
- So it is not recommended if the patient is pregnant.
- If a patient has an infection, giving them lifileucel can make the infection worse.
- So it is not recommended if the patient has an active infection.
-
Contraindication - A reason to avoid a medicine. -
Infection - When germs like bacteria or viruses enter our body and make us sick.

Lifileucel - Tumor infiltrating lymphocyte (TIL) therapy
Important lifileucel prescribing safety information.
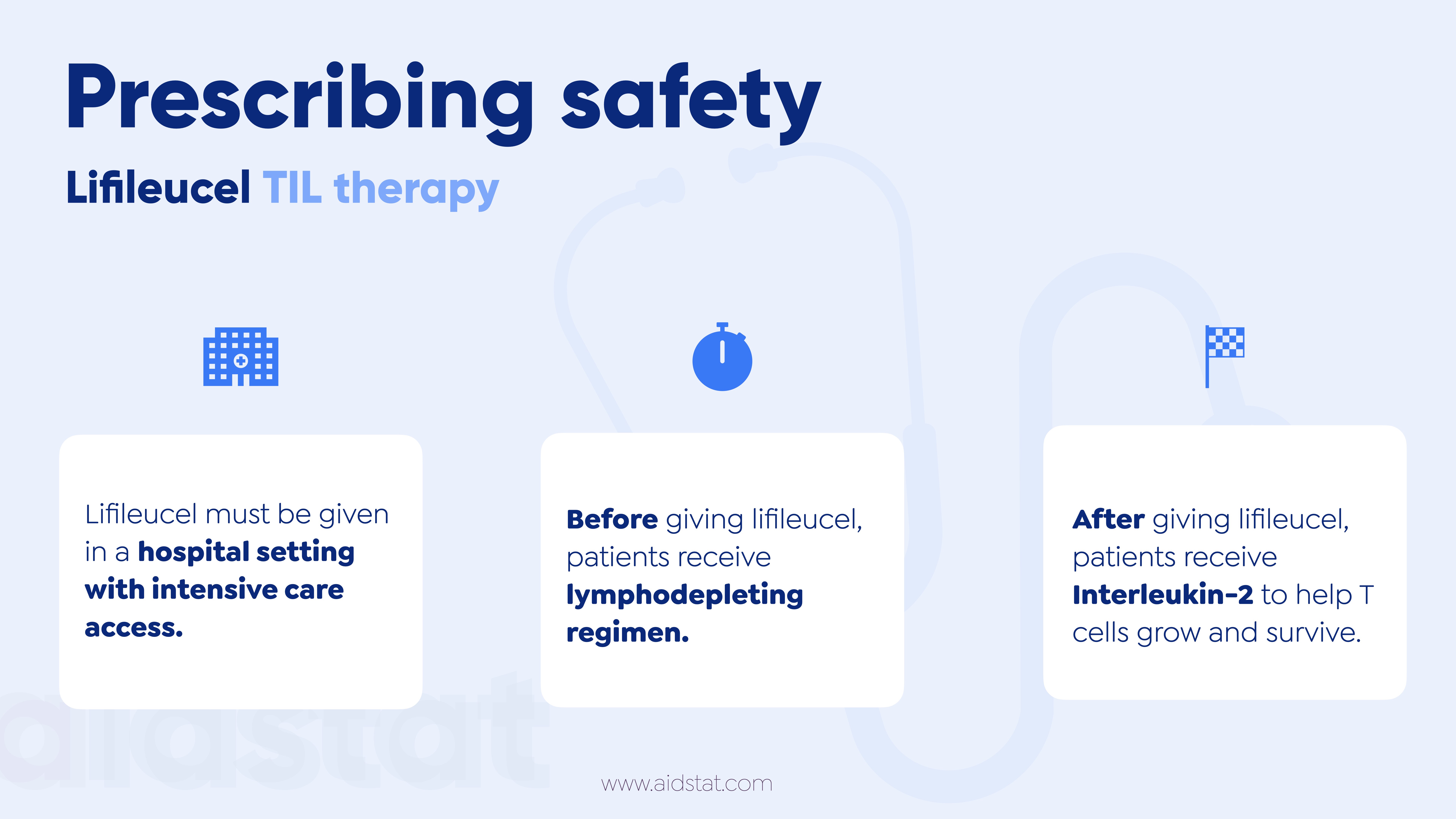
- Before giving lifileucel patients receive a lymphodepleting regimen.
- Lymphodepleting regimen gets the body ready for cell therapy.
- After lifileucel, patients are given Interleukin-2 (IL-2)
- IL-2 helps T cells survive, giving lifileucel the best chance to work against cancer.
-
Lymphodepleting regimen - A treatment that lowers certain white blood cells. It prepares the body for cell therapy. Without it the body might reject the new cells.
-
Interleukin-2 - A protein made by some white blood cells. It helps white blood cells grow and fight sickness better.

Lifileucel - Tumor infiltrating lymphocyte (TIL) therapy
Lifileucel patient counselling advice.
- Lifileucel treatment can weaken the immune system.
- Patients should be advised to avoid live vaccines for a few months after treatment.
- After treatment, patients should be advised to stay close to the treatment centre for at least a few weeks.
- This allows the healthcare team to monitor and act fast if needed.
-
Live vaccines - Contain active weakened germs which the body learns to fight without getting very ill. Live vaccines can cause serious sickness in weaker immune systems.
-
References
References
1. Iovance Biotherapeutics (n.d.). Highlights of Prescribing Information. [online] Available at: https://www.fda.gov/media/176417/download?attachment [Accessed 4 Jun. 2024]. Link
2. Iovance Biotherapeutics (n.d.). Patient Information AMTAGVI (lifileucel). [online] Available at: https://www.iovance.com/AMTAGVI_Patient_Information/ [Accessed 4 Jun. 2024]. Link
3. Parums, D.V. (2024). Editorial: First Regulatory Approval for Adoptive Cell Therapy with Autologous Tumor-Infiltrating Lymphocytes (TILs) – Lifileucel (Amtagvi). Medical science monitor, 30. doi:https://doi.org/10.12659/msm.944927. Link
4. Sarnaik, A.A., Hamid, O., Khushalani, N.I., Lewis, K.D., Medina, T., Kluger, H.M., Sajeve Samuel Thomas, Evidio Domingo-Musibay, Pavlick, A.C., Whitman, E.D., Martín-Algarra, S., Corrie, P., Curti, B.D., Judit Oláh, Lutzky, J., Weber, J.S., Larkin, J., Shi, W., Takamura, T. and Madan Jagasia (2021). Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. journal of clinical oncology, 39(24), pp.2656–2666. doi:https://doi.org/10.1200/jco.21.00612. Link
5. www.amtagvi.com. (n.d.). AMTAGVI (Lifileucel) Learn about AMTAGVI (Lifileucel). [online] Available at: https://www.amtagvi.com/receiving/ [Accessed 4 Jun. 2024]. Link